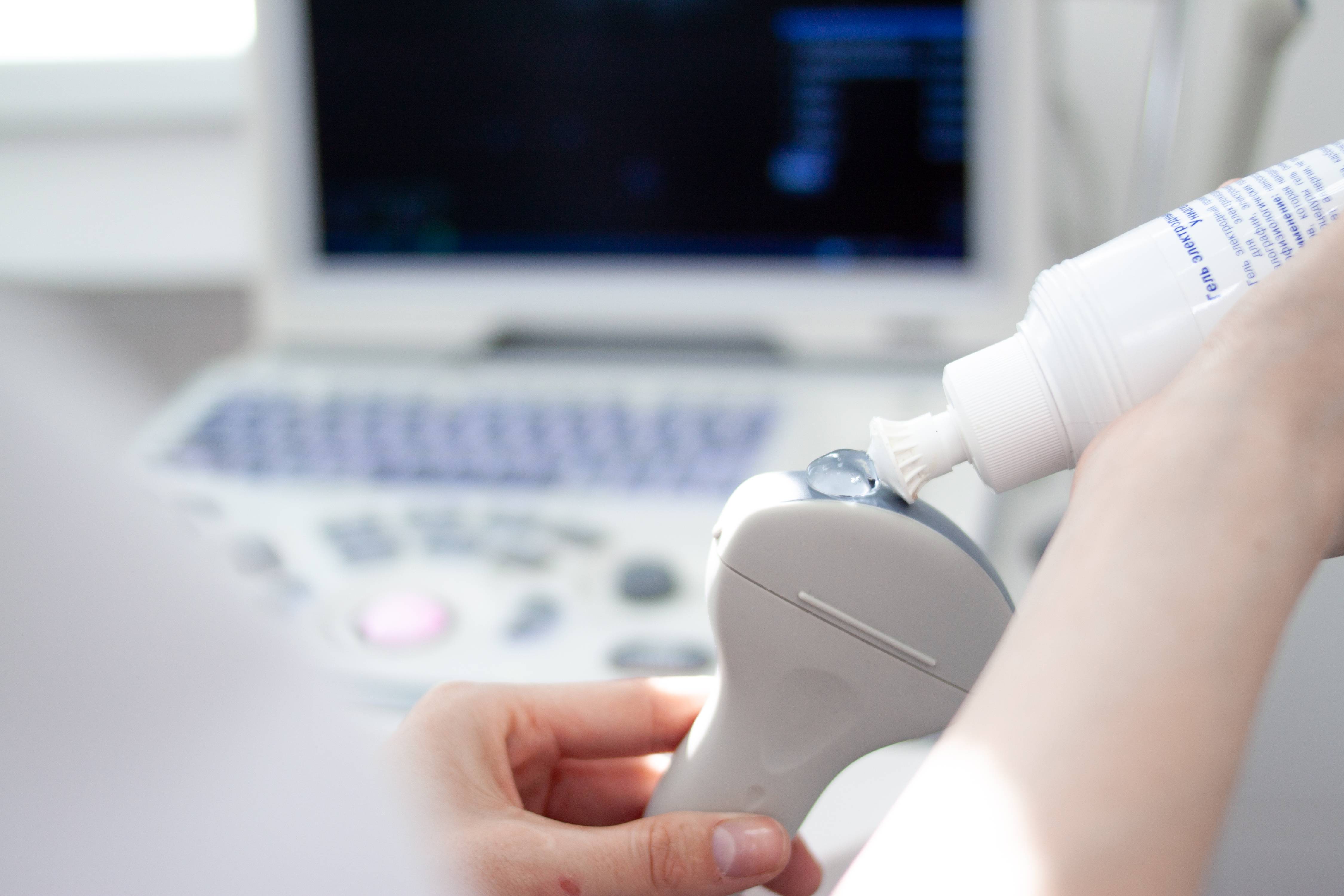
The UK Health Security Agency (UKHSA) has amended its guidelines on the use of sterile ultrasound gel.
The new guidance says that sterile gel should be used “if an invasive procedure is likely or planned on or near the site in the following 24 hours - this includes viewing or initial assessment’ of a site by ultrasound prior to undertaking an (aseptic) invasive procedure.
The UKHSA adds that “If an unplanned invasive procedure is indicated and undertaken within 24 hours of non-sterile gel use, then clean and prepare the site as indicated in the general principles of non-sterile ultrasound gel use section.”
It also says that “where there is contact with a mucous membrane (for example for transrectal, transvaginal or ophthalmic procedures) - note: sterile gel to be used on the outside of probe covers and inside the probe cover if manually filled” this is to cater for probe covers that are already pre-filled and care should be taken to avoid puncturing the probe cover if pre-filled.
Click here for the UKHSA guidance on Ultrasound gel - good infection control practice.