22-26 April 2024 is #GreenerAHP week
Learn how climate change is affecting health, how health systems are affecting climate, and what we can do to deliver environmentally sustainable healthcare.
Learn more


CPD Now
Your online portfolio and personal CPD accreditation. A tool where you can plan, record and reflect on your CPD activities.
Access CPD Now HereAccess our resources
The Society of Radiographers works in partnership with the College of Radiographers to deliver unique resources

Education and Career Framework for the Radiography Workforce
The fourth edition provides guidance for the education and career development of the radiography profession.

Engaging Managers
The new web section, featuring tool kits and hot topics, for those with line management responsibilities is now available.

Students
Information for those studying to be radiographic professionals
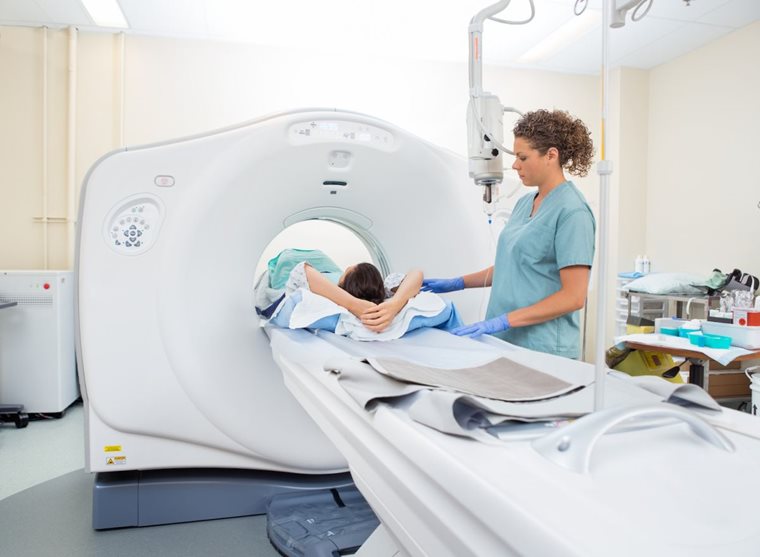
The Society of Radiographers
As a trade union and UK professional body for the diagnostic imaging and radiotherapy workforce, with our members, we shape policy and standards, pioneer new ways of working, and ensure safe and fair workplaces.
Contact Us


-(1).jpg?width=600&height=300&ext=.jpg&width=1200&resizemode=force)